In the study of thermodynamics and heat transfer, two fundamental concepts that often cause confusion among students and practitioners are specific heat and latent heat. While both concepts relate to heat transfer and energy changes in substances, they describe fundamentally different processes and have distinct characteristics that are crucial to understanding how materials behave when subjected to thermal energy.
The distinction between specific heat and latent heat is essential for engineers and scientists working in thermal engineering, power generation, refrigeration, and numerous other fields. Proper understanding of these concepts enables accurate analysis of energy systems, prediction of material behavior under different thermal conditions, and optimization of thermal processes for maximum efficiency.
Introduction to Heat Transfer Concepts
Before delving into the specific differences between specific heat and latent heat, it’s important to establish a foundation in the basic principles of heat transfer and thermal energy. Heat transfer is a form of energy transfer that occurs due to a temperature difference between systems or within a system.
Heat can be transferred through three primary mechanisms:
- Conduction: Transfer of energy through direct contact between particles
- Convection: Transfer of energy through the movement of fluids
- Radiation: Transfer of energy through electromagnetic waves
When thermal energy is added to or removed from a substance, it can manifest in two fundamentally different ways: as a change in temperature (sensible heat) or as a change in phase (latent heat). Understanding these two forms of heat transfer is crucial for analyzing thermal systems and predicting their behavior.
Specific Heat (Sensible Heat)
Specific heat, also known as sensible heat, refers to the amount of thermal energy required to raise the temperature of a unit mass of a substance by one degree without changing its phase. This concept is fundamental to understanding how materials respond to thermal energy input when they remain in the same physical state.
Definition
The specific heat capacity (often simply called specific heat) of a substance is defined as:
This definition emphasizes that specific heat relates to temperature changes within a single phase, distinguishing it from latent heat which involves phase changes.
Mathematical Formula
The relationship between heat transfer, mass, specific heat, and temperature change is expressed by the fundamental equation:
Where:
- Q is the heat energy transferred (Joules)
- m is the mass of the substance (kilograms)
- c is the specific heat capacity (J/kg·K or J/kg·°C)
- ΔT is the change in temperature (Kelvin or Celsius)
This equation shows that the amount of heat required to change the temperature of a substance is directly proportional to its mass, specific heat capacity, and the temperature change.
Process Characteristics
Heat transfer involving specific heat has several key characteristics:
- Temperature Change: The defining characteristic is that the substance’s temperature changes while its phase remains constant
- Continuous Process: As heat is added, the temperature increases continuously (or decreases if heat is removed)
- Reversible: The process is typically reversible – cooling the substance will return it to its original state
- Measurable: Temperature changes can be easily measured with thermometers or other temperature sensing devices
Latent Heat (Hidden Heat)
Latent heat, often called “hidden heat,” refers to the amount of energy absorbed or released by a substance during a phase change at a constant temperature. Unlike specific heat, which causes temperature changes, latent heat is associated with the rearrangement of molecular structures during phase transitions without any change in temperature.
Definition
Latent heat is defined as:
The term “latent” comes from the Latin word meaning “hidden,” reflecting the fact that this energy transfer does not result in a temperature change that can be easily detected by conventional thermometers.
Types of Latent Heat
There are several types of latent heat, corresponding to different phase transitions:
- Latent Heat of Fusion (L_f): Energy required to change a substance from solid to liquid phase (melting) or released when changing from liquid to solid (freezing)
- Latent Heat of Vaporization (L_v): Energy required to change a substance from liquid to gas phase (vaporization/boiling) or released when changing from gas to liquid (condensation)
- Latent Heat of Sublimation (L_s): Energy required to change a substance directly from solid to gas phase (sublimation) or released when changing from gas to solid (deposition)
Mathematical Formula
The relationship for latent heat transfer is expressed as:
Where:
- Q is the heat energy transferred during the phase change (Joules)
- m is the mass of the substance undergoing phase change (kilograms)
- L is the specific latent heat (J/kg)
This equation shows that the amount of heat required for a phase change is directly proportional to the mass of the substance, but independent of the temperature (which remains constant during the phase change).
Process Characteristics
Heat transfer involving latent heat has distinct characteristics:
- No Temperature Change: The temperature remains constant throughout the phase change process
- Energy Storage: Energy is either absorbed (endothermic process) or released (exothermic process) during the phase change
- Molecular Rearrangement: The energy is used to break or form intermolecular bonds rather than increase kinetic energy
- Constant Conditions: The process occurs at specific temperature and pressure conditions for each substance
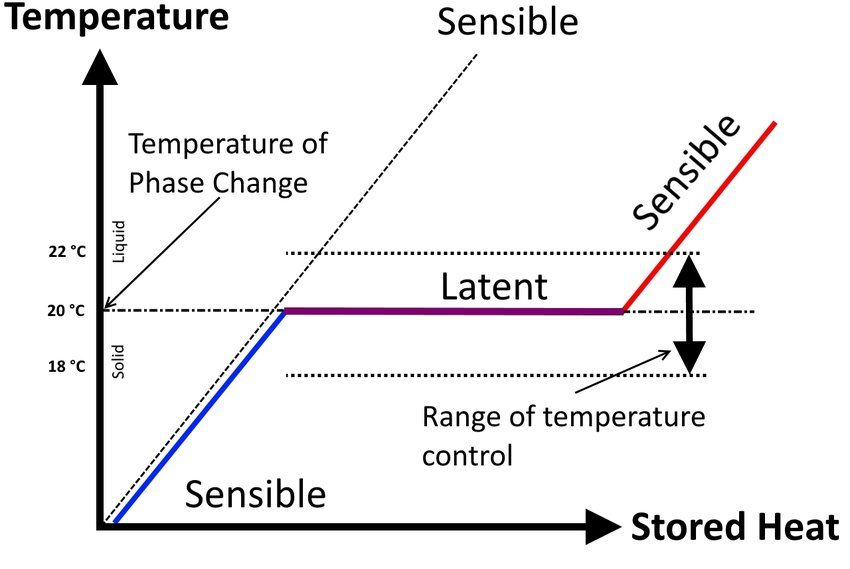
Graphical Representation
The differences between specific heat and latent heat are most clearly illustrated through graphical representations, particularly the heating curve for water, which shows how a substance behaves when heat is continuously added.
Heating Curve Analysis
A typical heating curve for water (or any pure substance) shows several distinct regions:
- Solid Heating (Ice): As heat is added to ice below 0°C, the temperature increases linearly – this represents sensible heat transfer where specific heat governs the process
- Melting Plateau: At 0°C, the temperature remains constant as ice melts to water – this represents latent heat of fusion
- Liquid Heating (Water): As heat is added to water between 0°C and 100°C, the temperature increases linearly – again, sensible heat transfer governed by specific heat
- Boiling Plateau: At 100°C, the temperature remains constant as water boils to steam – this represents latent heat of vaporization
- Gas Heating (Steam): As heat is added to steam above 100°C, the temperature increases linearly – sensible heat transfer in the gas phase
The sloped sections of the curve represent sensible heat transfer where the added energy increases the kinetic energy of the molecules, resulting in temperature rise. The flat sections represent latent heat transfer where the added energy is used to overcome intermolecular forces, changing the phase without temperature change.
Summary Comparison Table
To clearly illustrate the differences between specific heat and latent heat, let’s examine a comprehensive comparison table:
| Characteristic | Specific Heat (Sensible Heat) | Latent Heat (Hidden Heat) |
|---|---|---|
| Definition | Heat required to change temperature without phase change | Heat required for phase change at constant temperature |
| Formula | Q = mcΔT | Q = mL |
| Temperature Change | Yes – temperature changes continuously | No – temperature remains constant |
| Phase Change | No – substance remains in same phase | Yes – substance changes phase |
| Energy Destination | Increase in molecular kinetic energy | Breaking/forming of intermolecular bonds |
| Measurable with Thermometer | Yes – direct temperature measurement | No – temperature remains constant |
| Process Reversibility | Yes – cooling reverses heating | Yes – but in opposite direction |
| Typical Values (Water) | c = 4186 J/kg·°C (liquid) | L_f = 334,000 J/kg (fusion) L_v = 2,260,000 J/kg (vaporization) |
| Energy Requirement | Relatively small for moderate temperature changes | Very large – much more energy than sensible heat |
| Examples | Heating water from 20°C to 80°C Cooling metal in air |
Melting ice at 0°C Boiling water at 100°C Condensation on cold surface |
Practical Applications and Examples
Understanding the difference between specific heat and latent heat has numerous practical applications in engineering, science, and everyday life. Let’s explore some key areas where this knowledge is essential.
Thermal Energy Storage
Latent heat is extensively used in thermal energy storage systems because of the large amount of energy that can be stored or released during phase changes:
- Ice Storage Systems: Commercial buildings use ice storage for air conditioning, taking advantage of the large latent heat of fusion
- Phase Change Materials (PCMs): Materials that store and release thermal energy during phase transitions are used in building materials, clothing, and electronics cooling
- Heat Pipes: Devices that transfer heat efficiently using latent heat in evaporation and condensation processes
Refrigeration and Air Conditioning
Refrigeration cycles rely heavily on latent heat effects:
- Evaporator: Refrigerant absorbs latent heat of vaporization from the cooled space
- Condenser: Refrigerant releases latent heat of condensation to the environment
- Energy Efficiency: The large latent heat values allow for compact, efficient heat transfer
Power Generation
Steam power cycles utilize both specific heat and latent heat:
- Boiler: Water is heated (sensible heat) and then converted to steam (latent heat)
- Turbine: Steam expands, converting thermal energy to mechanical energy
- Condenser: Steam releases latent heat of condensation to become water
Everyday Applications
Many everyday phenomena demonstrate the principles of specific and latent heat:
- Sweating: Evaporation of sweat uses latent heat of vaporization to cool the body
- Cooking: Boiling water stays at 100°C regardless of heat input due to latent heat
- Weather: Cloud formation and precipitation involve latent heat effects
- First Aid: Ice packs use latent heat of fusion to absorb heat from injuries
Sample Problems and Calculations
Let’s work through some sample problems to illustrate the application of specific heat and latent heat concepts in practical calculations.
Problem 1: Combined Sensible and Latent Heat
Calculate the total heat required to convert 2 kg of ice at -10°C to steam at 120°C.
Given data:
- Mass of ice = 2 kg
- Initial temperature = -10°C
- Final temperature = 120°C
- Specific heat of ice = 2100 J/kg·°C
- Specific heat of water = 4186 J/kg·°C
- Specific heat of steam = 2010 J/kg·°C
- Latent heat of fusion = 334,000 J/kg
- Latent heat of vaporization = 2,260,000 J/kg
The process involves five steps:
- Heating ice from -10°C to 0°C (sensible heat)
- Melting ice at 0°C (latent heat)
- Heating water from 0°C to 100°C (sensible heat)
- Vaporizing water at 100°C (latent heat)
- Heating steam from 100°C to 120°C (sensible heat)
Step 1: Heating ice from -10°C to 0°C
Step 2: Melting ice at 0°C
Step 3: Heating water from 0°C to 100°C
Step 4: Vaporizing water at 100°C
Step 5: Heating steam from 100°C to 120°C
Total heat required:
This example clearly shows that the latent heat processes (melting and vaporization) require significantly more energy than the sensible heat processes, with vaporization alone accounting for more than 70% of the total energy required.
Problem 2: Cooling Process
Calculate the heat that must be removed to cool 500 g of steam at 120°C to ice at -20°C.
This is the reverse of the previous problem, involving the same processes but in reverse order with heat removal instead of addition. The calculations would be identical in magnitude but negative in sign, representing heat removal.
Factors Affecting Specific Heat and Latent Heat
Both specific heat and latent heat values can vary based on several factors. Understanding these dependencies is important for accurate calculations and predictions.
Factors Affecting Specific Heat
- Temperature: Specific heat can vary with temperature, especially over large temperature ranges
- Pressure: For gases, specific heat can be significantly affected by pressure changes
- Phase: Specific heat varies between solid, liquid, and gas phases of the same substance
- Molecular Complexity: More complex molecules generally have higher specific heats due to additional degrees of freedom
- Impurities: The presence of impurities can alter specific heat values
Factors Affecting Latent Heat
- Temperature: Latent heat values change with temperature, typically decreasing as temperature increases
- Pressure: Latent heat can be significantly affected by pressure, especially near the critical point
- Purity: Impurities can dramatically affect latent heat values and phase transition temperatures
- Molecular Structure: The arrangement and bonding in molecules affect the energy required for phase changes
Advanced Concepts and Considerations
Beyond the basic concepts, there are several advanced topics and considerations related to specific heat and latent heat that are important in specialized applications.
Heat Capacity vs. Specific Heat
While specific heat is the heat capacity per unit mass, heat capacity (C) is the total amount of heat required to raise the temperature of an entire object by one degree:
Where C is the heat capacity of the object. This distinction is important when dealing with objects of known mass rather than unit masses.
Molar Specific Heat
In chemistry and physics, it’s often more convenient to express specific heat on a molar basis rather than a mass basis:
Where C_m is the molar specific heat and M is the molar mass of the substance. This is particularly useful when dealing with chemical reactions and gas laws.
Degrees of Freedom and the Equipartition Theorem
The specific heat of gases can be understood through the equipartition theorem, which states that each degree of freedom contributes (1/2)R to the molar specific heat at constant volume:
- Monatomic gases: 3 translational degrees of freedom, C_v = (3/2)R
- Diatomic gases: 3 translational + 2 rotational degrees of freedom, C_v = (5/2)R
- Polyatomic gases: 3 translational + 3 rotational degrees of freedom, C_v = 3R
Critical Point Effects
Near the critical point of a substance, both specific heat and latent heat exhibit unusual behavior:
- Specific heat may become very large or even diverge
- Latent heat approaches zero as the critical point is approached
- The distinction between liquid and gas phases disappears
Measurement Techniques
Accurate measurement of specific heat and latent heat is crucial for engineering applications and scientific research. Various techniques have been developed for these measurements.
Specific Heat Measurement
- Calorimetry: Using a calorimeter to measure temperature changes when known amounts of heat are added
- Differential Scanning Calorimetry (DSC): Precise measurement of heat flow as a function of temperature
- Adiabatic Calorimetry: Measurements in thermally insulated systems
Latent Heat Measurement
- Bomb Calorimetry: For measuring heats of combustion and phase changes
- DSC for Phase Transitions: Precise measurement of latent heats during melting, crystallization, etc.
- Sorption Analysis: For measuring latent heats of adsorption and desorption
Conclusion
The distinction between specific heat and latent heat represents a fundamental concept in thermodynamics that has profound implications for understanding energy transfer and material behavior. While specific heat relates to temperature changes within a single phase, latent heat governs the energy requirements for phase transitions at constant temperature.
The mathematical relationships Q = mcΔT for specific heat and Q = mL for latent heat provide the quantitative tools needed to analyze thermal processes in engineering and scientific applications. The graphical representation through heating curves clearly illustrates how these two forms of heat transfer manifest in real-world situations.
The practical applications of these concepts are extensive and important. From thermal energy storage systems that utilize the large latent heats of phase change materials to refrigeration cycles that depend on latent heat effects for efficient operation, understanding these principles is essential for modern technology. The sample calculations demonstrate not only the mathematical procedures but also reveal the significant energy requirements for phase changes compared to temperature changes.
Advanced considerations such as the effects of temperature, pressure, and molecular structure on these properties, as well as specialized measurement techniques, show that the topic extends far beyond simple definitions. The behavior near critical points and the relationship to molecular degrees of freedom illustrate the deep connections between macroscopic thermal properties and microscopic molecular behavior.
In engineering practice, the proper application of specific heat and latent heat concepts is crucial for the design and optimization of thermal systems. Power plants, HVAC systems, chemical processing equipment, and countless other technologies rely on accurate understanding and application of these principles.
As we continue to develop more efficient energy systems and explore new materials for thermal management applications, the fundamental understanding of specific heat and latent heat remains as relevant as ever. Whether in the design of next-generation refrigerants, the development of advanced thermal storage materials, or the optimization of industrial processes, these concepts provide the foundation for innovation and improvement.
Students and practitioners should not only master the mathematical relationships but also develop an intuitive understanding of when and how these different forms of heat transfer occur. This deeper comprehension enables more effective problem-solving and system design in thermal engineering applications.
The large magnitudes of latent heats compared to specific heats, as demonstrated in the sample problems, highlight the tremendous energy storage and transfer capabilities of phase change processes. This understanding has led to the development of numerous technologies that exploit these effects for improved efficiency and performance.
In conclusion, the difference between specific heat and latent heat is more than a matter of definition – it represents two distinct mechanisms by which thermal energy interacts with matter, each with its own characteristics, applications, and significance in the natural world and engineered systems. Mastery of these concepts is essential for anyone working in fields related to thermal sciences and energy systems.